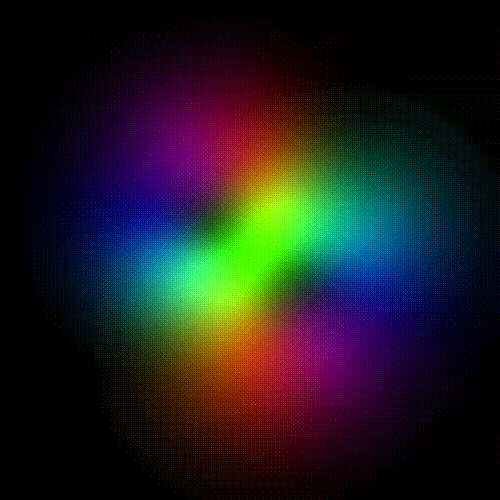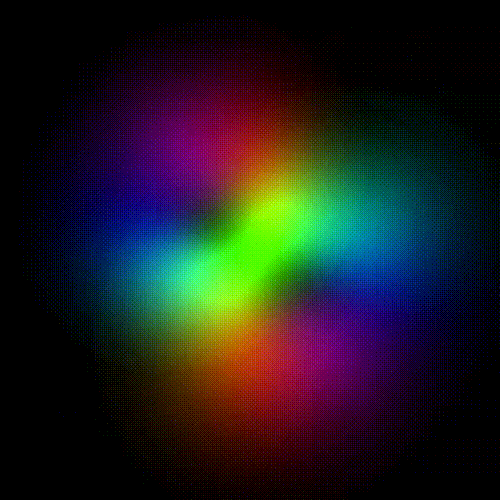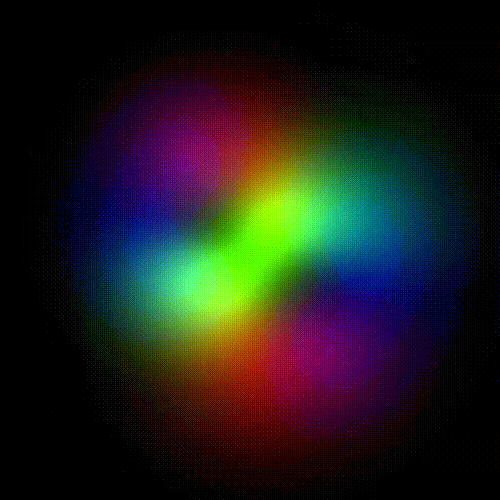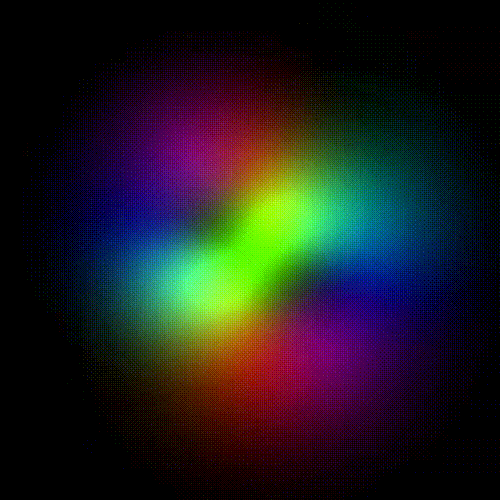Atomic orbital
xantox, 20 April 2008 in GalleryOther Languages:
Time evolution of an hydrogenic (single-electron) atomic orbital with quantum numbers | 3, 2, 1 > according to the Schrödinger equation (colors represent phase). In atomic matter, electrons orbiting the nucleus do not follow any determined classical path, but exist for each quantum state within an orbital, which can be visualized as a cloud of the probabilities of observing the electron at any given location and time.