Classical Molecules
Posted in: Gallery
Animation showing the interaction of four charges of equal mass1, two positive and two negative, in the approximation of classical electromagnetism. The particles interact via the Coulomb force, mediated by the electric field represented in yellow. A repulsive “Pauli force” of quantum mechanical origin, which becomes very large at a critical distance of about the radius of the spheres shown in the animation, keeps the charges from collapsing into the same point. Additionally, the motion of the particles is damped by a term proportional to their velocity, allowing them to “settle down” into stable (or meta-stable) states.
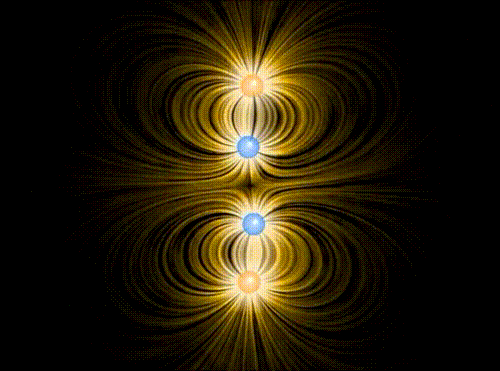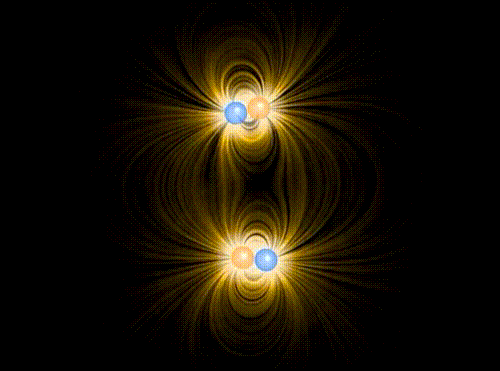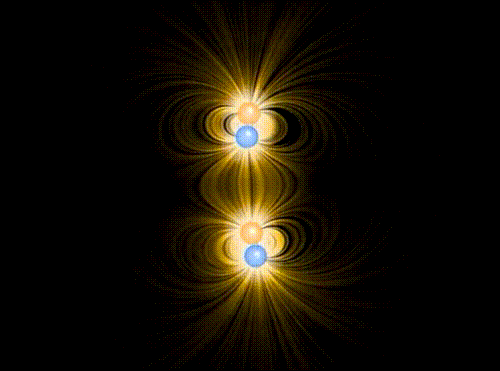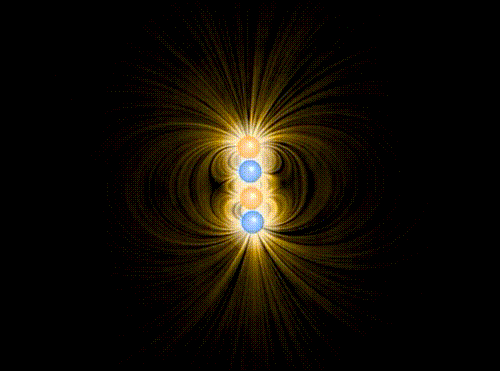
When the charges are allowed to evolve from the initial state, the first thing that happens (very quickly, since the Coulomb attraction between unbalanced charges is very large) is that they pair off into dipoles. Thereafter, there is still a (much weaker) interaction between neighboring dipoles (van der Waals force). Although in principle it can be either repulsive or attractive, there is a torque that rotates the dipoles so that it is attractive, eventually bringing the two dipoles together in a bound state. This mechanism binds the molecules of some substances into a solid.
- © 2004 MIT TEAL/Studio Physics Project, John Belcher [↩]
Return to: Classical Molecules
Social Web